04/16/2021
Developing Novel Microbial Platforms for Designer Ester Biosynthesis
Research paves way for creating useful chemicals with greater speed and flexibility.
Demonstration of efficiency and compatibility of the engineered AAT in an Escherichia coli whole-cell biocatalyst.
[Reprinted from Seo, H. et al. 2021 under a Creative Commons license (CC BY-NC-ND 4.0).]
The Science
Scientists use metabolic engineering and synthetic biology approaches to manipulate and control biocatalysts (enzymes and whole cells) to rapidly and efficiently produce useful chemicals from renewable plant feedstocks. Esters are one such class of compounds that can be used as flavors, fragrances, solvents, and drop-in biofuels. In nature, esters are produced by enzymes known as alcohol acyltransferases (AATs) that condense an alcohol and an acyl-CoA in a thermodynamically favorable reaction. Many metabolic engineering efforts have used eukaryotic AATs for microbial biosynthesis of esters, but these AATs lack sufficient robustness, efficiency, and compatibility with different microbes, thus limiting ester production.
The Impact
As part of research conducted by the DOE Center for Bioenergy Innovation, a team of scientists leveraged enzymes known as chloramphenicol acetyltransferases (CATs) to create a new platform for microbial biosynthesis of designer esters. The enzymes worked in different microbial hosts using a variety of pathways and could even function at high temperatures. This flexibility makes them more practical for industrial-scale manufacturing.
Summary
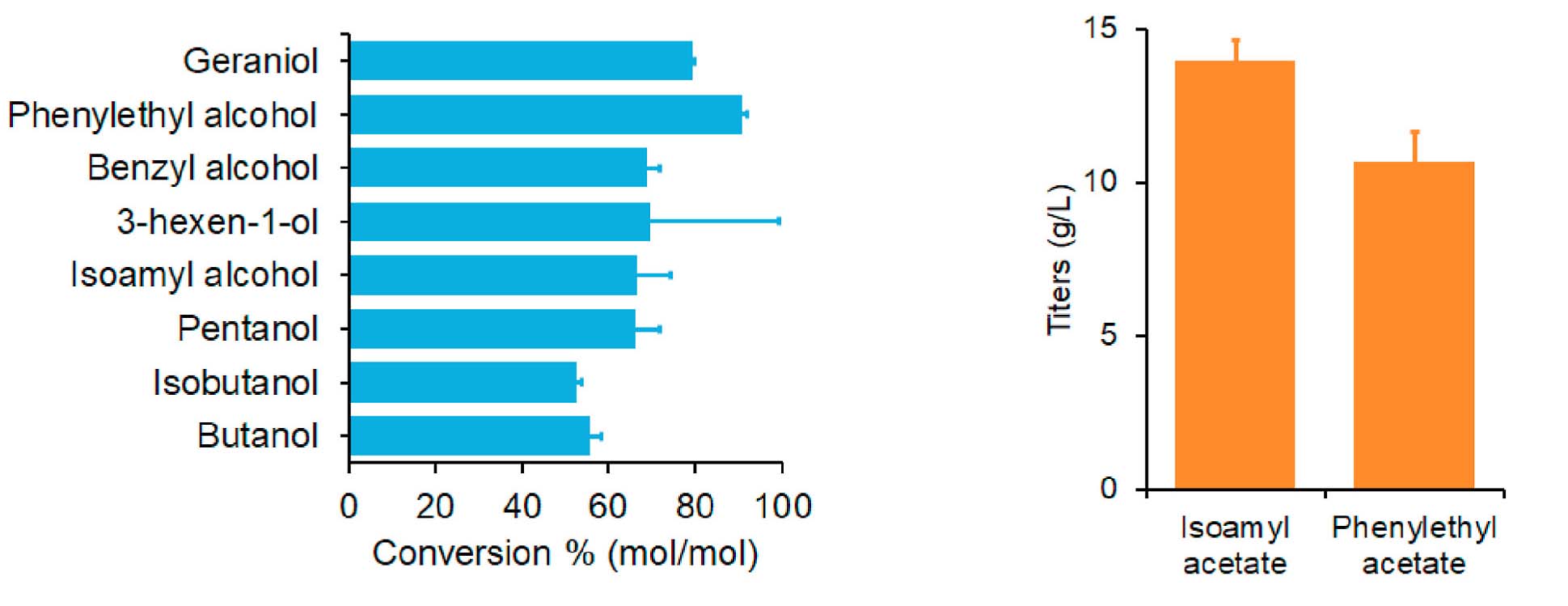
Through bioprospecting and model-guided protein engineering, researchers engineered CATs to function as robust and efficient AATs for microbial biosynthesis of linear, branched, saturated, unsaturated, and aromatic esters. The engineered AATs are thermostable and compatible with various pathways and microbial hosts, including mesophiles and thermophiles. The study demonstrated high conversion of various alcohols and achieved about 14 g/L of isoamyl acetate with >95% (mol/mol) conversion efficiency. This work not only presents a robust, efficient, and highly compatible AAT platform for designer bioester production, but also elucidates the impact of enzyme thermostability on engineering heterologous pathways in thermophiles.
Principal Investigator
Cong T. Trinh
University of Tennessee
[email protected]
Related Links
BER Program Manager
Kent Peters
U.S. Department of Energy, Biological and Environmental Research (SC-33)
Biological Systems Science Division
[email protected]
U.S. Department of Energy, Biological and Environmental Research (SC-33)
Biological Systems Science Division
[email protected]
Funding
This research was supported by the National Science Foundation CAREER award (NSF#1553250) and the Center for Bioenergy Innovation (CBI; DE-SC0019412), a U.S. Department of Energy (DOE) Bioenergy Research Center funded by the Biological and Environmental Research (BER) Program, within the DOE Office of Science.
References
Seo, H. et al. “Engineering Promiscuity of Chloramphenicol Acetyltransferase for Microbial Designer Ester Biosynthesis.” Metabolic Engineering 66, 179–190 (2021). [DOI:10.1016/j.ymben.2021.04.005]
Lee, J.-W. et al. “Probing Specificities of Alcohol Acyltransferases for Designer Ester Biosynthesis with a High-Throughput Microbial Screening Platform.” Biotechnology and Bioengineering 118(12), 4655–4667 (2021). [DOI:10.1002/bit.27926]
