Deep Chemical Imaging of the Rhizosphere
Authors:
Marcus T. Cicerone1* ([email protected], PI), Abigail Diering1, Madison Green1, Rajas Poorna1, Caroline Filan1, Lily S. Cheun1, Joel E. Kostka1, Francisco E. Robles1,2
Institutions:
1Georgia Institute of Technology; 2Emory University
Abstract
Beneficial diazotrophic microbes promote plant growth and productivity by consuming sugars and other compounds exuded by roots and, in turn, provide fixed nitrogen to the plant. Although a large fraction of the carbon fixed by plants during photosynthesis is secreted through roots to sustain the root microbiome, this metabolic exchange is not well understood. Understanding the nitrogen-carbon nexus will help to develop transformative biofertilization technologies that require a smaller carbon commitment from plants to the nitrogen-fixing microbes. This research team is building a label-free microscope to image metabolic activity and chemical exchange between plants and bacteria deep within thick living plant roots and their associated rhizosphere microbial communities.
In year three, the team continued to develop quantitative phase and broadband coherent Raman imaging modalities for quantifying dynamic and chemical aspects of the carbon-nitrogen nexus in the rhizosphere. The team used quantitative oblique back-illumination microscopy (DqOBM) to image bacterial culture of three different diazotrophs (Sinorhizobium meliloti, Azotobacter vinelandii, and Rahnella aquatilis) cultured on media. Results from these imaging experiments were compared with DqOBM images from the root cap and elongation zone of Arabidopsis thaliana primary roots that had been inoculated with either A. vinelandii or R. aquatilis the primary root system. This study’s findings demonstrate that quantitative dynamic phase imaging can effectively characterize microbial dynamics and provide insights into plant–microbe interactions in situ.
Trends in dynamics observed with DqOBM were consistent with a dependence of energy level on carbon and nitrogen, and the team demonstrated strong linear correlations between the nitrogen fixation and the DqOBM signal energy. Since the signal energy level measured by DqOBM is a sum of all microbial activities, it is necessary to have orthogonal information to directly link dynamics observed by imaging with rates of growth or nitrogen fixation.
Researchers also used broadband coherent anti-Stokes Raman imaging to identify spectral changes in bacteria under the same conditions where DqOBM was used. Raman spectra of S. meliloti are obtained during lag, exponential, and stationary growth phases under varying media conditions designed to induce nitrogen fixation. Known Raman signatures of general metabolic activity and nitrogen fixation were correlated to these culture conditions.
Additionally, broadband coherent anti-Stokes Raman scattering (BCARS) was used to image Medicago truncatula nodules that host S. meliloti in the infection thread and nodule zones where bacteria exhibit characteristics of these different growth and media conditions. Researchers used SampleMap—a variant of uniform manifold approximation and projection the team adapted for Raman imaging—to map the Raman signatures obtained from the Medicago nodules to phenotypes expressed in the culture experiments. These SampleMaps will ultimately be compared to high-resolution spatial transcriptomic maps of sectioned root nodules whose sister serial slices have been imaged with BCARS.
Image
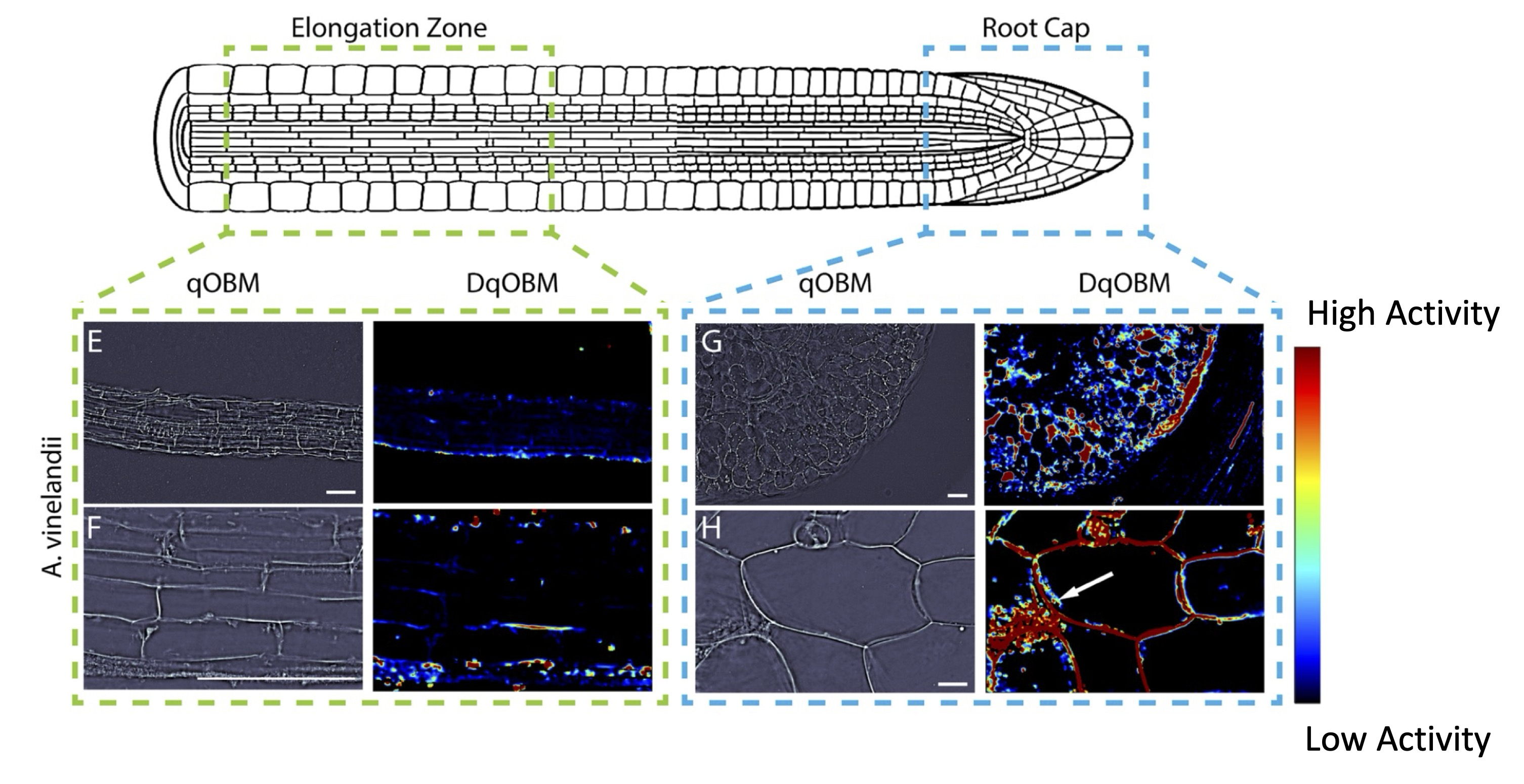
Arabidopsis thaliana Roots Inoculated with Azotobacter vinelandii. (E,F) The elongation zone with minimal dynamics. (G,H) Increased activity in the root cap. [Adapted from Filan, C., et al. 2024. "Label-Free Functional Analysis of Root-Associated Microbes with Dynamic Quantitative Oblique Back-Illumination Microscopy,' Scientific Reports 14, 5812. DOI:10.1038/s41598024-56443-1. Republished under Creative Commons license (CC BY 4.0)]
