Fluorescence Lifetime-Based Imaging of Bacillus subtilis Membrane Potential
Authors:
Debjit Roy1* ([email protected]), Xavier Michalet2, Kiran Bharadwaj1, Evan W. Miller3, Shimon Weiss1,4 (PI)
Institutions:
1UCLA-DOE Institute for Genomics and Proteomics; 2University of California–Los Angeles; 3University of California–Berkeley; 4Bar-Ilan University
Abstract
Membrane potential (MP) changes can provide a simple readout of bacterial functional and metabolic state or stress levels. While several optical methods exist for measuring fast changes in MP in excitable cells, there is a dearth of such methods for precise (and calibrated) measurements of steady-state MPs in bacterial cells. Conventional electrode-based methods for the measurement of MP are not suitable for small bacterial cells. Existing optical electrophysiological techniques based on fluorescent Nernstian probes have been successfully used in many studies, but they do not provide precision or absolute quantification of MP or their changes. This team presents a novel, calibrated MP recording approach to address this gap.
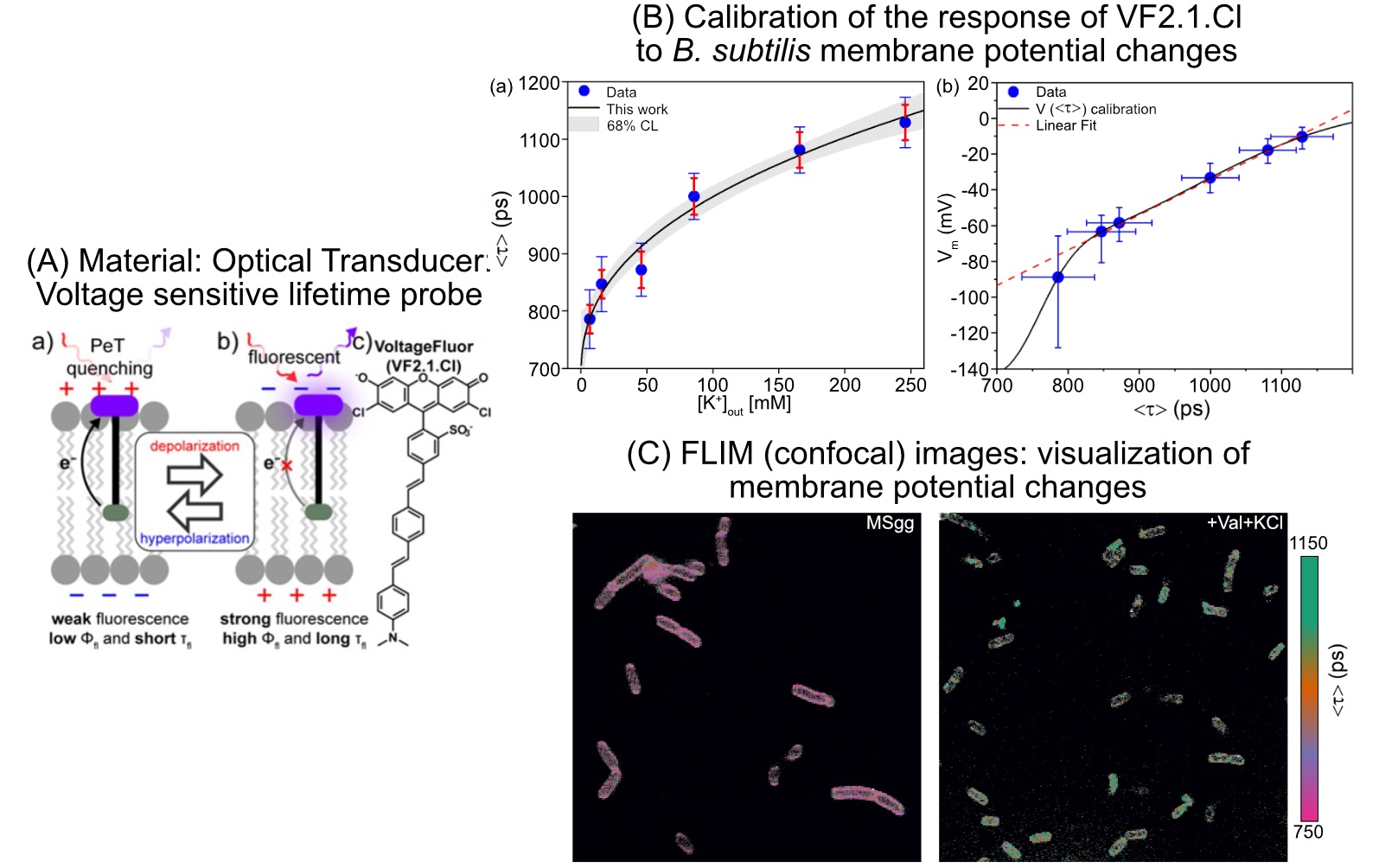
This group’s method uses (1) a unique optical transducer (a chromophore wire-donor construct), that utilizes intrinsic photoinduced electron transfer (PeT) mechanism to measure MP via its fluorescence lifetime and (2) a quantitative fluorescence lifetime imaging microscopy (FLIM) data analysis based on phasor analysis. In order to visualize individual bacterial cells’ MPs under different extracellular conditions, amplitude-averaged lifetime maps were computed from pixel-wise phasor fractions. This allows group members to accurately measure even small MP changes in single bacterial cells.
Calibration of membrane potential estimation via phasor-FLIM measurements has been achieved by modulating MP artificially through changing ionic (potassium +) concentration gradients across the membrane utilizing ionophores. Applying this technique to Bacillus subtilis, researchers estimated their normal MP at -86 millivolts and a chemically modulated depolarized state at +1 mV. This breakthrough work paves the way for deeper insights into bacterial electrophysiology and bioelectricity research.
Image
Fluorescence Lifetime-Based Imaging of Bacillus subtilis Membrane Potential [Courtesy University of California–Los Angeles and Bar-Ilan University]
