Novel Multimodal Chemical Nano-Imaging Technology to Visualize and Identify Small Biomolecules Exchanged in Microbial Communities
Authors:
Scott Lea1* ([email protected], PI), Patrick El-Khoury1, Sarah Akers1, Edo Aprà1, Victoria Orphan2
Institutions:
1Pacific Northwest National Laboratory; 2California Institute of Technology
Goals
The goal of this project is to develop bioimaging technologies that will significantly advance understanding of microbial metabolism and communication in real space. This is an outstanding challenge because existing approaches do not have the required spatial resolution. This project will combine nano-optics tools with multimodal (non)linear optical spectroscopy to identify and image small biomolecules with nanometer spatial resolution under physiological conditions. Specifically, the team plans to enhance the spatial resolution in optical extinction, Raman/fluorescence, and coherent Raman/ two-photon fluorescence/second-harmonic generation spectroscopy down to ~1 to 2 nanometers under ambient conditions to visualize metabolites involved in a wide range of microbial and plant processes.
Abstract
Though linear nano-optical measurements have been demonstrated, nonlinear nano-optical measurements comprise a novel high risk, high reward aspect of this project. Nonlinear nano-optical measurements, while providing improved signal-to-noise ratios, are challenging when chemical imaging and identification of biomolecules is the goal as this requires spectrally resolved detection schemes, e.g., in coherent Raman-based vibrational nanoimaging and nanospectroscopy. These coherent nano-optical measurements, however, require long collection times that restrict their usefulness in a point scanning hyperspectral nanoimaging scheme where full-time traces must be recorded at every position. The team plans to overcome this difficulty by decreasing collection times by orders of magnitude using time-series analysis in combination with machine learning.
With these measurements, an entirely novel set of nanoscopic selection rules is expected. Team members are developing a theoretical framework to assign experimental observables—primarily the linear and nonlinear optical signatures of biomolecules—by coupling ab initio molecular dynamics computed optical spectra to classical finite difference time-domain simulations to reproduce experimental plasmon-enhanced spectral nanoimages.
As these simulations are time-consuming, the team will apply machine learning and time-series analysis to dramatically accelerate these simulations. To optimize and validate the performance of this technology for bioimaging applications, this research team will benchmark the system using environmental consortia of anaerobic methane-oxidizing archaea and syntrophic partner sulfate-reducing bacteria. Using these consortia, this technology will allow researchers to spatially resolve the electronic and vibrational signatures of large multiheme cytochromes embedded in the extracellular matrix, thereby providing the first direct evidence that these proteins predicted in the genomes are exported into the extracellular space.
While this current effort is devoted to optimization of this multimodal nanoimaging technology capable of in-liquid operation, the team is also developing novel nano-optical methods, including (1) ultralow frequency tip-enhanced Raman scattering (Wang et al. 2023a) and (2) broadband extinction nanoimaging and nanospectroscopy (Wang et al. 2023b). The first approach not only allows tracking the chemical identities of bioanalytes, but also enables tracking crystallinity on the nanoscale. Further developments would allow this method to mature into a nano-optical analogue of X-ray diffraction. The second approach is a novel measurement that boasts subnanometer spatial resolution under ambient laboratory conditions. Initial measurements tracked spatially varying plasmon resonances throughout the formation of a junction plasmon. This effort sheds light on the fundamental mechanisms behind optical nanospectroscopy and nanoimaging, which is important for multimodal spectral nanoimaging of biomolecules. More generally, the use of the probe as a nanoscopic broadband light source will allow measuring the nano-extinction spectra of yoctomolar concentrations of biomolecules.
Image
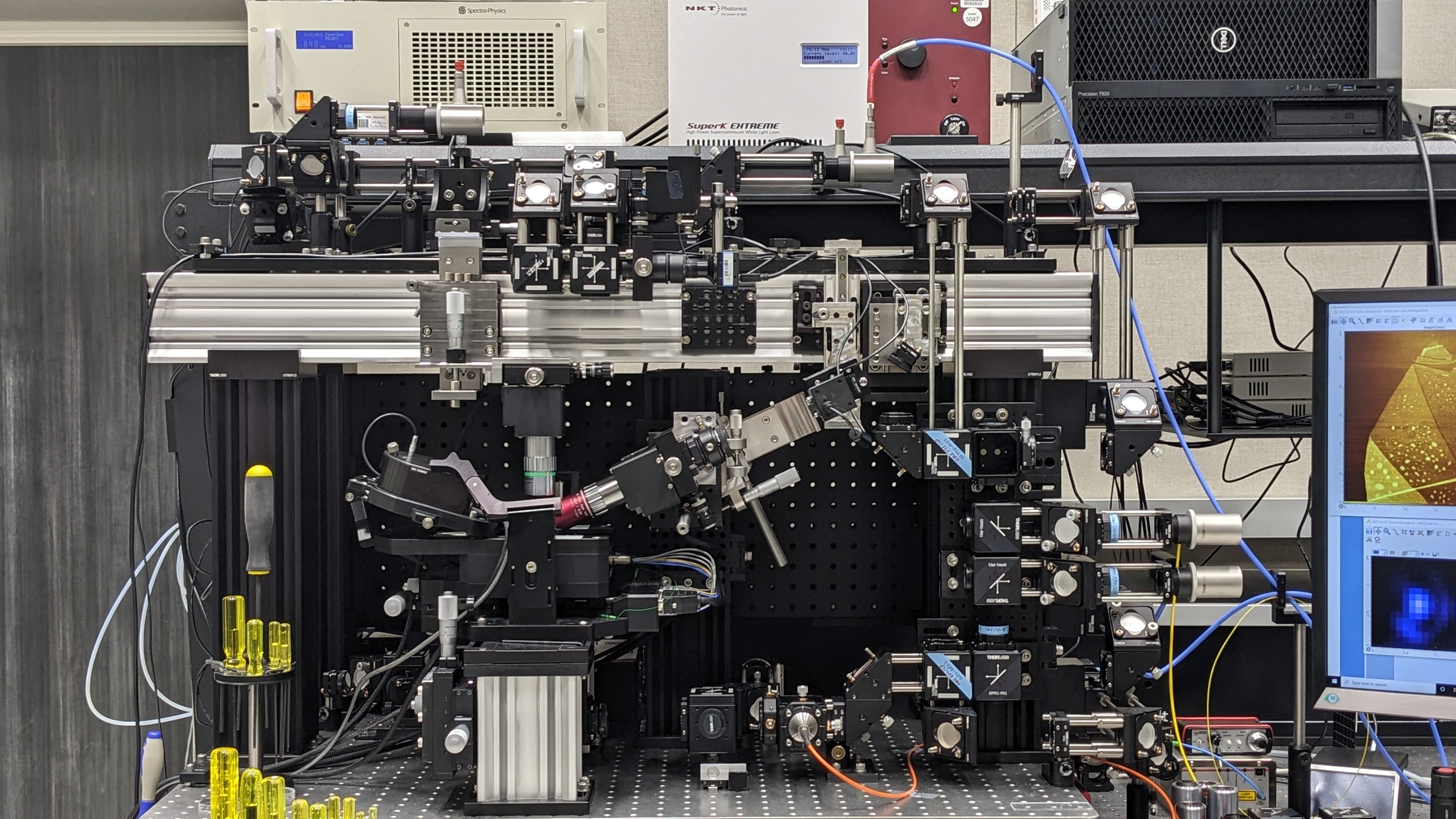
BIGTUNA. Instrumentation showing the AFM (lower left) and associated light sources and optics. The instrumentation provides top-, side- and bottom-access to the sample using numerous linear and nonlinear near-field imaging modalities (as described in the text) for nanoscale biochemical imaging. [Courtesy Pacific Northwest National Laboratory]
References
Wang, C.-F., et al. 2023a. “Probing Local Optical Fields via Ultralow Frequency Raman Scattering from a Corrugated Probe,” Journal of Physical Chemistry Letters 14, 8334–8. DOI:10.1021/acs.jpclett.3c02122.
Wang, C.-F., et al. 2023b. “Subnanometer Visualization of Spatially Varying Local Field Resonances that Drive Tip- Enhanced Optical Spectroscopy,” Nano Letters 23(19), 9114–8. DOI:10.1021/acs.nanolett.3c03028.
