Ultra-Sensitive High-Resolution Label-Free Nonlinear Optical Microscopy for Imaging Plant–Microbe Interactions In Vivo
Authors:
Na Ji1* ([email protected], PI), Trent Northen2, John Vogel2
Institutions:
1University of California–Berkeley; 2Lawrence Berkeley National Laboratory
Goals
Root biology is pivotal in addressing global challenges including sustainable agriculture and climate change. However, roots have been relatively understudied among plant organs, partly due to the difficulties in imaging root structures in their natural environment. This team aims to develop advanced optical microscopy techniques to reduce photodamage and improve imaging resolution and depth for live plant roots and microbes grown in microfabricated ecosystems (EcoFABs).
Abstract
Researchers have succeeded in using microfabricated ecosystems (EcoFABs) to establish growing environments with optical access and employing nonlinear multimodal microscopy of third-harmonic generation (THG) and three-photon fluorescence (3PF) to achieve label-free, in situ imaging of live roots and microbes at high spatiotemporal resolution.
THG has enabled researchers to observe key plant root features in mature and meristem roots including laminar structures down to the vasculature, Casparian strips, dividing meristematic cells, root cap and border-like cells, as well as resolve subcellular features including nuclear envelopes, nucleoli, starch granules, and putative stress granules (see figure, p. 48). THG from the cell walls of bacteria and fungi also provides label-free contrast for visualizing these microbes in the root rhizosphere. With simultaneously recorded 3PF fluorescence signal, the team has achieved single-bacterium tracking and subcellular imaging of fungal spores and hyphae in the rhizosphere, indicating this method’s potential for studying plant–microbe interactions.
To improve image resolution, the team combined adaptive optics with THG microscopy. By measuring and correcting sample-induced optical aberrations on the excitation light, adaptive optics has led to a substantial increase in root THG signal.
The group is now working on optimizing homodyne-mixing setups for both THG and second harmonic generation (another coherent light scattering process that provides structural contrast without external labels) microscopy. By interfering a larger reference harmonic signal with the harmonic signal generated by the sample, researchers can enhance the detectability of the sample signal thus reduce the amount of light needed for harmonic generation and its associated photodamage.
Image
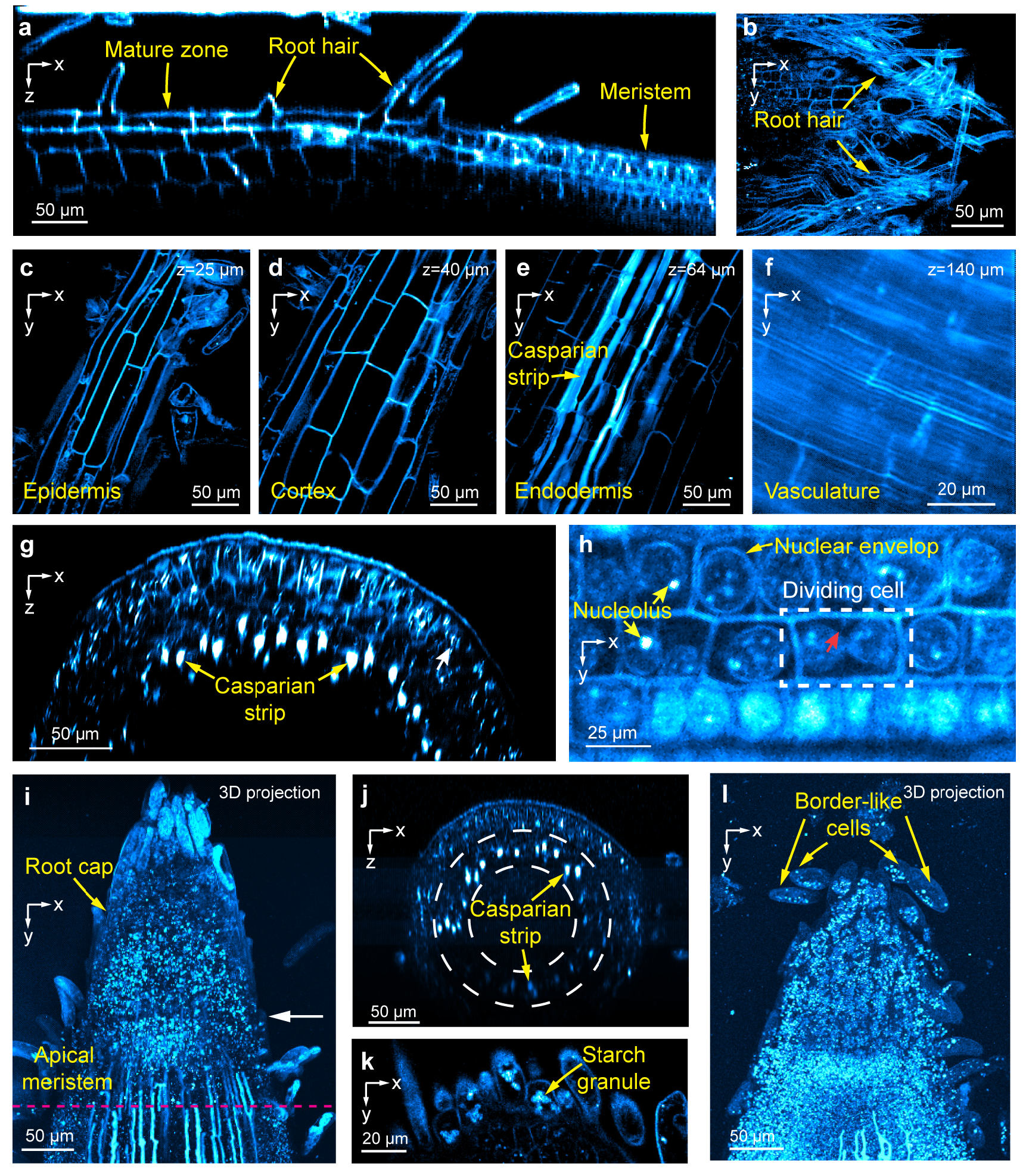
Label‐Free Structural Imaging of Live Brachypodium distachyon Roots Using Third‐Harmonic Generation Microscopy. (a) Axial (xz) image of a root from mature zone to meristem. (b) Lateral (xy) images of structural features in the mature root including (c) root hairs enveloping root surface, (d) epidermis, (e) cortex, (f) endodermis, and (g) vasculature of mature root. (h) Axial image of meristem root and (i) lateral view of subcellular structures. Red arrow: Emerging cell wall. Images of root tips, including (j) brightest-spot projection with depth cueing (100% to 50%) of a 230-micrometer (μm)-thick image stack through a root tip, (k) axial image of apical meristem along the pink line in i, (l) root cap cells, and brightest-spot projection with depth cueing (100% to 50%) of an 88-μm-thick image stack of a root tip. Post-objective power of 1300 nanometer excitation light: (a) 3 milliwatts; (b) 4 milliwatts; (c-e) 2.6 milliwatts; (f) 14 milliwatts; (g) 5.3 milliwatts; (h) 7 milliwatts; (i-j) 3 to 15 milliwatts; (k) 4 milliwatts; (l) 2.4 to 2.8 milliwatts. [Modified from Pan, D., et al. 2024. "Label-Free Structural Imaging of Plant Roots and Microbes Using Third-Harmonic Generation Microscopy," bioRxiv. Preprint. DOI:10.1101/2024.04.13.589377. Republished under Creative Commons license (CC BY-NC-ND 4.0)]
